By: Sarah Solomon
I have always been fascinated by the natural world –
by the species that I encounter on a daily basis, and by those that exist on
faraway lands. In thinking of how complicated and diverse human population
dynamics can be, I’ve always sought to understand how other species’
populations are regulated. Why do some species go extinct, and what prevents
others from meeting this same fate?
With ever-increasing human activity around the globe,
some species are actually beginning to flourish beyond their natural ranges. From
Asian carp to Dog-strangling Vine, the increased abundance and distribution of
native and introduced species can be detrimental to the survival of others.
 |
| Louis Charles Birch, 1918-2009 University of Sydney |
In the early 1940s, Australian insect ecologist Louis
Charles Birch began to study how certain species of Dacinae fruit flies were becoming pests as a result of the expansion
of cultivated fruit crops. Birch and his supervisor (and would-be lifelong
colleague) Herbert Andrewartha were fascinated by the relationship of evolution
to ecology, with a particular interest in how evolution can be charted in
dynamic species of insects.
At
the time of Birch and Andrewartha’s original research on the five most abundant
species of fruit flies in eastern Australia, there was a prevailing hypothesis
suggesting that all animal populations were self-regulating. This meant that
populations of animals would increase under favourable conditions, and that
eventually the population would grow so crowded that the birth rate would drop
and the death rate would increase – hence the notion of self-regulation. It was
believed that once a population reached a low enough density, the pressures on
it would decrease and the population would be spared from extinction. Now in a
laboratory setting, there was compelling evidence to support this hypothesis,
but Birch had a different idea.
What actually happens to populations in nature? Why don’t they become
extinct? How might we regulate populations of species that have extended beyond
their natural ranges? Birch was part of a generation that started to wonder
about these notions as they related to changes in natural environments due to
human activities. He decided to look both within and outside of different fruit
fly species in order to explore how external factors affect species density and changes
in distribution.
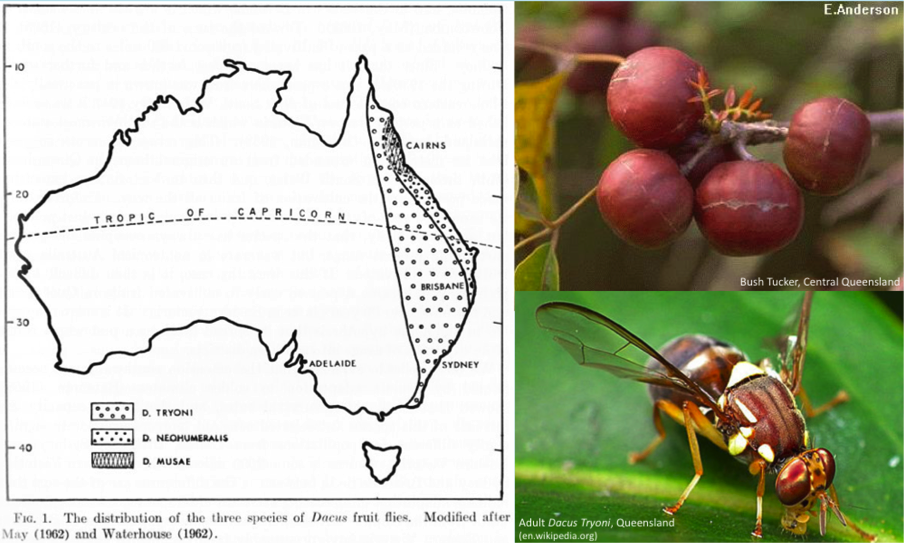
In particular, D.
tryoni had become a pest to fruit crops spanning the eastern coastal
portion of Australia, with a significant increase in its range, adapting to cooler
southern temperatures. He was also interested in charting this change – are
species actively adapting to environmental changes, and are these changes
observable? As it turns out, D. tryoni
was even hybridizing with D. neohumeralis
– another endemic fruit fly species of eastern Australia – to produce a
population of flies with a 15 percent higher survival rate of immature eggs
than D. tryoni. And all of this as a
result of cultivating more fruit crops across the country!
Thus it seems that Birch was on to something – as it
turns out, something very important indeed. It is unclear whether Birch could
have anticipated just how much of an
impact human activity would have on the environment, and in turn, just how much
this change would affect species population dynamics. Today, conservation is at
the forefront of science and policy, and the notion of studying the effects of
abiotic factors on species population dynamics is imperative.
With scientists like Birch paving the way for
thinking beyond the “self-regulating” hypothesis, research groups like that of
Australian ecologist Euan Ritchie are committed to producing population models
that can help inform conservation policies, and protect at-risk species from
extinction.
In a 2009 study, Ritchie and colleagues uncovered how
competition between the antilopine wallaroo and its wide-spreading counterpart,
the Eastern grey kangaroo, is a threat to the survival of the former species.
They also found that habitat – especially changes to landscape and the
introduction of cattle ranching – contributed greatly to the viability of these
species, and that of the common wallaroo (http://www.ncbi.nlm.nih.gov/pubmed/19175695).
This type of modeling research is commonplace in the field of ecology today, as
the notion of abiotic factors playing a significant role in species' survival is
a widely accepted school of thought. With the growing impacts of climate change
becoming more and more evident each day, it seems we have come a long way from
the era when Birch’s ideas were considered a minority view.
More than fifty years later, the need to produce
sound science with which to inform conservation policy is critical. Since
Birch’s kick-start to understanding population dynamics, significant
advancements have been made in genetics, and in the technologies for analyzing
genetic diversity. Such techniques are helping to further highlight the types
of genetic adaptations that Birch started to chart in the 1950s, producing
fascinating insights into how populations are disappearing, appearing and
adapting to external changes.
Overall, have we made all that much progress since
Birch?
I would like to think that in many ways we have, but
we still have yet to bridge the gap that exists between sound science and policy
enforcement. It seems that despite strides being made on the scientific
forefront, useful data are often not used or are discounted by policy
decision-makers to suit the goals of various stakeholders. Research, like that
of Birch and Andrewartha, and more currently Ritchie and colleagues, has major
implications for conservation issues, and particularly for the growing concerns
of harmful invasive species (see The Genetics of Colonizing Species).
Share your views, and leave a comment below!
References:
Baker, H. G., and Stebbins, G. L. (1965). The genetics of colonizing species: proceedings. Academic Press Inc.
Ritchie, E. G., Martin, J. K., Johnson, C. N., and Fox, B. J. (2009). Separating the influences of environment and species interactions on patterns of distribution and abundance: competition between large herbivores. Journal of Animal Ecology, 78, 724-731. doi: 10.1111/j.1365-2656.2008.01520.x