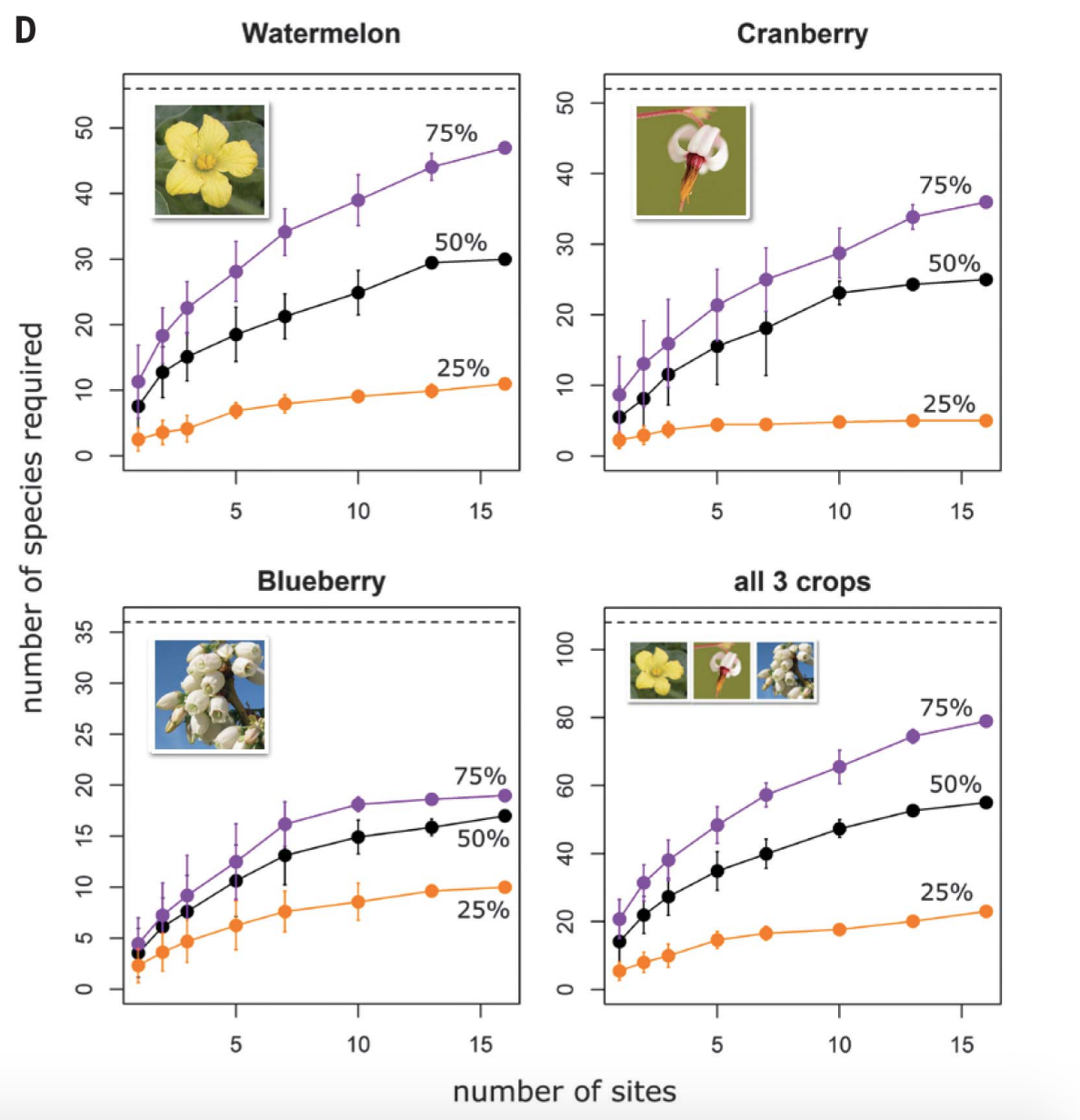
Unless you’ve been living under a
rock (much like native mining bees in Canada), you’ve probably seen the
numerous campaigns to “Save the Bees”. Bee species across the globe are in
decline. There are many factors that contribute to this decline, such as
pesticide use, colony collapse, disease, habitat loss, and climate change1.
Many of these factors interact with one another, exacerbating the consequences
and impacts. Conservation efforts are being implemented to try to stop the loss
of these pollinators, and the valuable services they provide to humans. Canada
is no exception. There are local, provincial, and national policies and
programs operating and currently being developed in order to reduce the impacts
of these threats. In the past few years, programs like The Bee Cause, Bees Matter, Feed
the Bees, and others have
implemented programs and recommendations in order to increase the bee
populations in Canada. Honey Nut
Cheerios has even campaigned
to get the public engaged and involved in the conservation of bees. These
programs, however, all have one common issue: they focus their efforts
on Honey Bees.
 |
An example of a campaign by Honey Nut
Cheerios, focusing on honey bees.
|
There are no
native honey bees in Canada. The most well-known bee in Canada was not even
present in the country until it was introduced from Europe in the 1600s2.
The European Honey Bee was intentionally introduced to Canada for honey
production, and since has increased in number dramatically, both in farmed and
wild colonies. Honey bees have large colonies, allowing them to be easily
managed and farmed. They also pollinate crops and produce honey, which may make
them seem more economically valuable than their native, non-honey-producing
counterparts. However, there have been unexpected impacts of the introduction
of the European Honey Bee on native bee species in Canada.
There
are over 800 native bee species in Canada. While there are many different types
of bees in Canada, the best understood group of native bees are bumble bees.
Bumble bees have the ability to buzz pollinate, which allows them to obtain
pollen from plants with pollen that is difficult to extract. Many of these
plants are economically valuable, such as kiwi and blueberry crops. This, along
with general pollination, makes managed populations of bumble bees worth
several billion dollars annually3. Bumble bees naturally have low
genetic diversity and can be subject to inbreeding depression, leading to
declining populations and making the some species more vulnerable to extinction4.
Threats can then interact with these low population levels, and intensify population
loss.
 |
A male Rusty-patched Bumble Bee, one
of Canada’s native bee species. It is currently listed as endangered in Canada.
|
Aside from
facing the same threats as honey bees, native bumble bees are also threatened
by the very presence of honey bees. Competition for resources with honey bees
is a major threat to native bumble bees. A study performed in the United
Kingdom found that bumble bees at sites with high honey bee density were
significantly smaller in body size when compared to their relatives at sites
with low honey bee density5. An additional study discovered a
reduction of native bumble bee colony success when colonies were experimentally
exposed to honey bees6. Honey bees generally produce larger colony
sizes which can store a larger amount of resources than bumble bees. They also
have the ability to communicate with one another about valuable floral resource
locations7. Honey bees have a larger foraging range than native
bumble bees, and have an increased ability to forage on introduced plant
species7. These adaptations allow honey bees to outcompete native
bumble bees, and commandeer sparse resources in the area.
Threats
from honey bees do not just end at competition; pathogens and parasites
specifically adapted to honey bees have been shown to have the ability to spread
to wild bumble bee populations. Managed honey bees are known to carry higher
than natural levels of pathogens8, which can be transmitted to wild
bumble bee populations when the bees interact. In particular, two pathogens
endemic to honey bees, C. bombi and N. bombi, are wreaking havoc on bumble
bee populations. While these pathogens do not have lethal effects, their
sublethal effects can be devastating to colonies. These pathogens cause reduced
pollen loads, a decline in flowers visited per minute, slower growth rates of
colonies, decreased queen reproductive rates, shortened life spans and diminished
colony growth8. With small populations already, entire bumble bee
colonies can be wiped out by these pathogens. Honey bee parasites, such as the
Small Hive Beetle, have also been shown to be able to spread to bumble bee
colonies, where they consume the wax, pollen, and nectar stores of hives8.
While honey bees have co-evolved with these parasites and pathogens for eons,
bumble bees have not had the time to adapt to these threats, making them much
more vulnerable to these hazards.
 |
Small Hive Beetle infestation in a
honey bee colony.
|
But why do we care about losing
native bees? The same concerns about the loss of honey bees applies to native
bees. Native bee species pollinate crops and flowers, which we depend on for
food. It is estimated that about one in three
bites of food we consume can be traced back directly to pollination by bees
and other pollinators. However, native bees have been found to be more
effective pollinators than honey bees. Some plant species in Canada rely
solely on native bees for their pollination. With the loss of native bees,
these plants will also become endangered, along with many other food crops
requiring pollination. Additionally, there is a severe lack of research into native
bees. Research tends to focus on honey bee populations, resulting in much more
knowledge of honey bee behaviours, adaptations, actions, and responses to
stressors. The truth is, we don’t know much about native bee species in Canada.
We have no idea what the consequences of the loss of these species will be.
However, this does not excuse us from protecting these bees. If anything, this
lack of knowledge should increase our urge to protect them, so we have the opportunity
to learn about them in the future.
The
native bee species in Canada share little life history traits with the European
Honey Bee8, making many conservation efforts that focus on honey
bees unsuccessful. Focusing conservation efforts on one species may not
address the specific needs of native bees. In addition, by focusing on
improving honey bee populations, there will be increased stress on native bees,
which will lead to a decline in their populations. If we continue with these
conservation strategies, we may threaten native species further.
An
increase in honey bee populations will increase parasite and pathogen levels in
native bees, and also increase the competition between honey bees and native
bees. So what can you do to focus conservation efforts on native Canadian bees?
For starters, avoid the use of pesticides, which will decrease already low
populations8. Improve your knowledge of bee species, and report
invasive or introduced species in areas used by native bee species. Plant a
wide variety of native
plants with high pollen and nectar concentrations to ensure newly emerging
bees have the resources they need to survive. And finally, avoid tilling,
mowing, or burning in areas where native bee species, particularly ground
dwelling species, are known to live. With increased knowledge of native bee
needs, and species specific conservation efforts, it is hoped that native bee
species will begin to rebound. Let’s BEE positive!
BEE Informed – To get
involved with native bee conservation check out these links:
BEE-bliography:
1. Pettis, J.S., and K.S. Delaplane. 2010. Coordinated
responses to honey bee decline in the USA. Adipologie
41:256-263.
2.
van Engelsdorp, D., and M.D. Meixner. 2010. A
historical review of managed honey bee populations in Europe and the United
Sates and the factors that may affect them. Journal
of Invertebrate Pathology 103:80-95.
3.
James, R., and T.L. Pitts-Singer. 2008. Bee Pollination in Agricultural Ecosystems.
Oxford University Press, USA.
4.
Zayed, A., and L. Packer. 2005. Complementary
sex determination substantially increases extinction proneness of haplodiploid
populations. Proceedings of the National
Academy of Sciences of the United States of America 102:10742-10746.
5.
Goulson, D., and K. Sparrow. 2009. Evidence for
competition between honey bees and bumble bees: Effects on bumble bee worker
size. Journal of Insect Conservation 13:177-181.
6.
Thomson, D. 2004. Competitive interactions
between the invasive European honey bee and native bumble bees. Ecology 85:458-470.
7.
Goulson, D. 2003. Effects of introduced bees on
native ecosystems. Annual Review of
Ecology, Evolution, and Systematics 34:1-26.
8.
Colla, S.R. 2016. Status, threats and
conservation recommendations for wild bumble bees (Bombus spp.) in Ontario, Canada: a review for policymakers and
practitioners. Natural Areas Journal 36:412-426.
Image Sources:
1. https://bringbackthebees.ca
2. https://inaturalist.com
3. http://beeaware.org.au/archive-pest/small-hive-beetle/#ad-image-0